NATCO PHARMA LIMITED (INDIA)
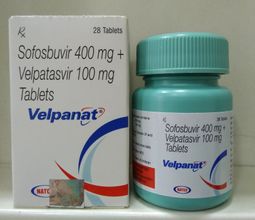
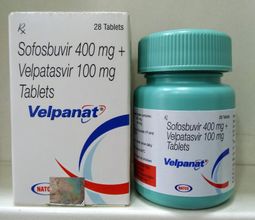
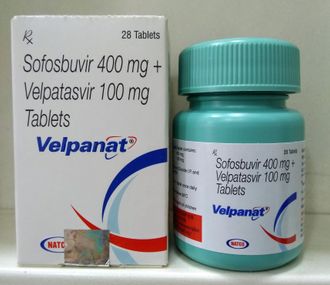
Sofosbuvir & velpatasvir-velpanat
“Sofosbuvir & Velpatasvir” are the Drugs / Molecules content in “Velpanat.
Sofosbuvir & Velpatasvir has high overall cure rates of 99% for GT2 patients and 95% for GT3 patients. Sofosbuvir & Velpatasvir was also found to be highly effective in patients with GT1, GT4, GT5 and GT6, with overall cure rates ranging from 97–100%.
Indication
Sofosbuvir & Velpatasvir are helpful for treatment of all Hepatitis C adults who are suffering from genotype 1-6 chronic hepatitis C virus.
Note
SIDE EFFECTS :
- Common side effects include muscle pains, headache, nausea, rash, diarrhea, insomnia and cough.
- More severe reactions are connected with allergic reactions to the medications and cardiovascular problems.
INTERACTIONS :
- For the HIV combination Efavirenz / Emtricitabine / Tenofovir, which reduces the area under the curve (AUC) of Velpatasvir by about 50%, and the CYP3A4 and Pgp inducer Rifampicin, which reduces its AUC by about 80%, rendering it likely ineffective.
- Digoxin is eliminated by Pgp; its AUC is increased by about 30% in combination with Velpatasvir and Sofosbuvir.
- Substances that reduce gastric acid, such as Antacids, H2 blockers, and Proton pump inhibitors, reduce Velpatasvir AUC by 20–40%.
OVERDOSE :
- Do not use more than prescribed dose.
- If you suspect you may have overdosed, call your healthcare provider.
- Do not take extra dose to make up for the missed dose.
Precaution
- Sofosbuvir & Velpatasvir are the prescription drug and should be used under proper medical guidance and advice.
- Caution should be exercised in patients with history of stomach problem, liver, kidney, or heart disease during pregnancy and breastfeeding.
Strength
400 mg Sofosbuvir / 100 mg Velpatasvir
Packing
1 X 28 Tablets (Plastic Container)
Storage
Store at room temperature.